My group at SRI Biosciences is driven by the idea of a “magic bullet” drug that can home in on a particular cell type and avoid healthy cells. This notion of a magic bullet has been around for 100 years, but finding molecules that recognize the address of a particular cell is challenging. In other words, how does a molecule distinguish between one cell type and another, such as cancerous cells versus healthy ones?
Cancer is an obvious application for this kind of targeted therapy. The side effects of chemotherapy—severe gastrointestinal problems, hair loss, immune suppression—result because most of these drugs work on all cells, but have more of an effect on cancer cells, which are rapidly dividing. Patients are given chemotherapy drugs not at the most efficacious level, but at the maximum tolerated dose to kill the cancer.
The current gold standard for specific cell targeting of cancerous tumors is the use of engineered immune molecules known as monoclonal antibodies. While these are very specific and will attach selectively to cancer cells, there are many disadvantages to using them. Monoclonal antibodies are large and difficult to modify chemically (such as by adding a drug), and they can be very expensive. A typical round of treatment with monoclonal antibodies for a cancer patient might cost $100,000 (usually covered by a patient’s health insurance).
We wanted to find something smaller, something that we could chemically modify in a specific fashion and attach whatever we wanted to it—but still retain the ability to recognize one cell type over another and specifically bind to those cells that could be created quickly and less expensively than monoclonal antibodies.
The concept: If we could take a chemotherapy drug and connect it to a molecule that would find and attach to cancer cells, it could then be delivered preferentially to those specific cancer cells and thereby decrease the side effects. This strategy could potentially allow for delivery of more drug to the right place than conventional methods since the toxic compounds are only affecting cancer cells rather than every cell in the body.
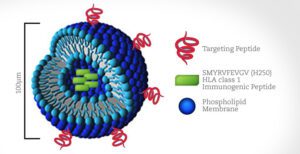
To find molecules that specifically target cancer cells, my lab has taken advantage of a technique called phage display. It utilizes phage, essentially a virus that attacks bacteria, to produce massive amounts of potential targeting agents quickly. We have engineered phage to produce short pieces of proteins called peptides. We incubate hundreds of millions of phage with these peptides displayed on their surface together with cancer cells—my lab focuses on non-small cell lung cancer—to see which ones bind to the cells. Anything that sticks can be sequenced to determine the composition of the peptide and then develop ways to use it.
We are exploring a range of applications of these peptides. Some focus on delivering drugs; some focus on doing molecular imaging to better understand the underlying biochemistry. We are also working with other groups at SRI to develop in vitro diagnostics to help detect cancer earlier or to follow treatment to see how a disease or therapy is progressing, pointing toward more personalized and precise medicines in the future. The latest news is that we are now using these peptides as immunotherapy. I recently presented an update of this work at the Essential Protein Engineering Summit (PEGS) held in Boston.
One of my research colleagues had the idea that, since we have identified peptides which bind to cancer specifically, what if instead of a drug we delivered an antigen that the immune system could recognize as foreign? If we delivered an antigen that people have been vaccinated against, maybe we could trigger a potent memory immune response that flags the cancer cells to be killed by the immune system naturally.
We created microscopic 100-nanometer “fat spheres” called liposomes and encapsulated inside each one a small peptide from measles that is known to cause an immune response to the measles vaccine. On the outside of the liposomes we put various cancer protein peptides that we have identified by phage display screening so that liposomes carrying this antigen would be delivered specifically to cancer cells.
Basically, we are trying to trick the immune system into thinking that cancer cells are infected with measles without actually using measles virus. If the body’s immune cells encounter a cancer cell with this measles protein on its surface, they will kill those cancer cells. We have found that there is a significant reduction in tumor growth rate using the targeted liposome treatment in mice, so our hypothesis appears to be correct.
We have a lot more work to do, finding different antigens and studying how this process is working, and also studying it in multiple different cancer types to see if it is broadly applicable. Overall we think using our identified peptides as immunotherapy is a promising idea, and our search for a magic bullet will continue.
Research reported in this SRI Blog post related to the Essential Protein Engineering Summit was supported by National Cancer Institute & National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under grant number R01CA164447 & R01EB014244 respectively.


