Recently, researchers in SRI Biosciences’ Center for Immunology and Infectious Diseases, in collaboration with colleagues at UK-based Sareum Ltd., a drug discovery company, identified a small molecule that looks like a promising therapy for psoriasis, an autoimmune disease of the skin affecting approximately two percent of the population. Symptoms of the condition often include painful, itching or burning and raised reddish patches on the skin that can range in severity from mild to debilitating and even life-threatening, depending on the type of psoriasis.
Our potential psoriasis therapy targets cytokines, which are molecules produced by cells to communicate with other cells and tell them what to do. Usually cytokines are produced in response to an insult, such as an invading pathogen. Inflammation occurs when the production of cytokines is not properly regulated and immune cells are overly activated.
While inflammation is usually a response to pathogens, sometimes it is triggered in the absence of a foreign invader. Inflammatory disorders result when the immune system, which normally protects the body, turns against it and causes damage to its own tissues. A large number of diseases have a basis in inflammation mediated by cytokines, especially autoimmune conditions such as rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis and psoriasis.
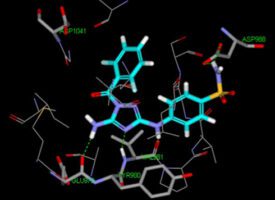
We found this promising molecule by searching for something that would deactivate cytokines by blocking the workhorses of the cytokine communication process: molecules known as Janus kinases (JAKs). The JAK family of enzymes facilitates the action of most cytokines and consists of JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2). JAK inhibitors have been explored as therapies that shut off errant immune system signaling in disease, and several have been FDA-approved. Our potential therapy is a selective TYK2 inhibitor that retained some activity against JAK1.
Among the JAK family members, TYK2 has yet to receive much attention from pharmaceutical companies developing JAK inhibitors. I thought TYK2 could be of interest as a potential new drug target because it is critical for the action of pro-inflammatory cytokines.
My goal was to eliminate pathogenic inflammation caused by harmful cytokines without affecting any other potentially beneficial cytokine signals.
I began searching for others working on TYK2 as a target for inhibition and found Sareum. With expertise in molecular design Sareum was eager to expand into autoimmune and inflammatory diseases with SRI Biosciences as a collaborator.
Once the research idea was planted, the biggest problem my research team and I faced was that JAKs are very similar in structure, meaning that any small molecule that inhibits one of the JAKs will likely inhibit others as well. JAK inhibitors currently on the market are also cross-reactive, so we needed something that would more specifically block the JAKs we targeted.
Even with expertly designed compounds from Sareum, a molecule completely selective for TYK2 was hard to achieve. Our most promising candidate was selective for TYK2 and JAK1. Based on my knowledge and understanding of autoimmune diseases, one of the diseases that I thought could benefit from both TYK2 and JAK1 inhibition was psoriasis, since the cascade of events leading to a psoriasis episode involves cytokines that require both of these JAKs.
Using the standard preclinical psoriasis model, we tested a molecule called SAR-20347 and discovered that it caused a striking decrease in disease symptoms. This TYK2/JAK1 inhibitor interrupted the psoriatic cascade of events and led to reduced activation of keratinocytes (skin cells that multiply excessively in psoriasis) and a reduction of pro-inflammatory cytokine levels.
Detailed results were presented at the Federation of Clinical Immunology Societies (FOCIS) meeting in Chicago in June 2014 and published in the Journal of Immunology.
While we are still in the early stages of research, our success so far is encouraging, and we plan to pursue other autoimmune and inflammatory diseases that might be treated by TYK2 inhibition. We hope to expand our TYK2-inhibitor program to address other autoimmune diseases, such as sepsis, rheumatoid arthritis and multiple sclerosis.
Further information about SRI’s TYK2 inhibitor program and other topics involving inflammation research will be presented at the Inflammation Research Association 18th Annual Conference being held at SRI headquarters in Menlo Park, California, on September 16-17, 2014.


