Citation
Rubio, M., Suarez, A., del Río, D., Galindo, A., Alvarez, E., & Pizzano, A. (2009). Rhodium Complexes with Pincer Diphosphite Ligands. Unusual Olefin in-Plane Coordination in Square-Planar Compounds. Organometallics, 28(2), 547-560.
Abstract
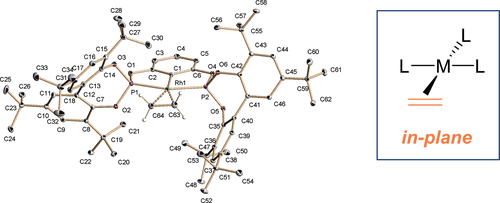
A family of rhodium complexes bearing pincer diphosphite (PCP) and neutral L (L = PPh3, CO, CNXy, C2H4) ligands has been prepared and characterized. Reactions between Rh(Cl)(PPh3)3 and the diphosphites 2 lead to the chloro hydrides Rh(H)(Cl)(PCP)(PPh3) (4), which can be deprotonated to yield the complexes Rh(PCP)(PPh3) (5). In the latter, the PPh3 ligand is labile and can be exchanged by CO, CNXy, and C2H4 to give derivatives 6−8. Most noteworthy is the fact that ethylene complexes 8a,b show a remarkable in-plane (ip) conformation in the solid state. Solution NMR studies for these complexes show fast olefin rotation in all the temperature ranges, while solution and solid-state 13C{1H} NMR experiments give practically superimposable spectra, also indicating the prevalence of the ip conformer in solution. Complementary detailed DFT calculations performed with models of compounds 8 indicate that the olefin conformational preference is due to a dual combination of steric effects arising from the reduction of the P−Rh−P angle from 180°, caused by pincer ligand chelation and by the nature of the phosphite substituents, which in the case of t-Bu groups perfectly outline a cavity for an ip coordination of the olefin.