SRI scientists collaborate with Lawrence Berkeley National Laboratory to chart a path for obtaining regulatory approval for a clinical development plan for a novel decorporation agent.
THE CHALLENGE
Exposure to harmful radionuclides as the result of a nuclear accident or terrorist attack can cause devastating human health effects1. A number of agents that remove heavy metals from the body are currently approved to treat radionuclide contamination. However, these decorporation agents, including Diethylene Triamine Pentaacetic Acid (DTPA) and Prussian blue, are effective against specific radionuclides and DTPA is currently administered intravenously. Ideal decorporation agents would address the broadest possible range of radionuclides, have demonstrated efficacy and safety in each, and be available in an oral formulation.
THE SOLUTION
Through more than 50 years of discovery effort, the Lawrence Berkeley National Laboratory (Berkeley Lab), a U.S. Department of Energy (DOE) laboratory managed and operated by The Regents of the University of California, has identified a number of decorporation ligands that can effectively chelate actinides/lantinides. Of these, 3,4,3-LI(1,2-HOPO) has been selected to be the most promising candidate to treat radionuclide contamination. In early efficacy studies, this “HOPO” agent demonstrated superior activity across a greater range of radionuclides compared to currently available decorporation therapies.
Given the scope of the HOPO project, government funding to support the development of this product is critical. Over the past eight years, SRI worked with Berkeley Lab to secure the necessary funding from a variety of government agencies, including NIH-ARRA, NCI-RAID, NIAID, and BARDA.
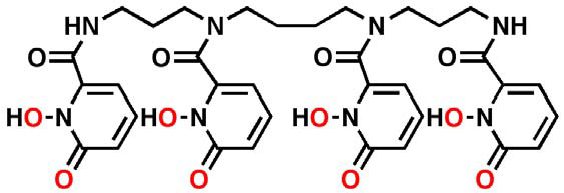
Background
Since field trials to define human efficacy of the HOPO product are not feasible, well-controlled animal efficacy and safety studies were used to provide substantial evidence of decorporation effectiveness and safety as required by the U.S. FDA’s Animal Efficacy Rule. As there is currently no precedent for the approval of any new radionuclide decorporation product under the Animal Efficacy Rule, SRI is charting new territory in obtaining regulatory approval for a clinical development plan under the Rule.
How it works
Berkeley Lab engaged SRI Biosciences (SRI) to develop the candidate HOPO ligands. SRI has organizational familiarity with the pre-clinical development pathway, proven expertise in radiation biology, demonstrated in-depth experience with regulated studies required to advance the HOPO program, and a breadth of services and capabilities.
Over a period of more than 8 years, Berkeley Lab and SRI worked together to:
- Identify and apply for government funding support;
- Develop strategies and timelines to ensure achievement of project deliverables and goals;
- Complete all the federally required safety studies (including pharmacokinetics, ADME, in vitro and in vivo safety studies);
- Develop the most appropriate analytical methods for dose analysis and stability testing;
- Develop bioanalytical methods in animal and human matrixes;
- Interact with U.S. Food and Drug Administration
- Manage regulatory filing for Investigative New Drug (IND)
- Develop the FDA-approved clinical protocol
- Initiate manufacturing of clinical Drug Product
Validation
SRI Biosciences has helped Berkeley Lab achieve critical milestones to move the HOPO compounds toward clinical development. These include:
- Ensuring the feasibility of large-scale compound manufacture
- Generating nonclinical safety and pharmacokinetics data required to support an Investigational New Drug (IND) application
- Developing a Phase 1 clinical trial protocol
- Securing FDA approval to proceed with the Phase 1 single ascending-dose first-in-human clinical trial. This represents Berkeley Lab’s first IND application for this class of compounds
Partner with us
SRI Biosciences partners with clients to seamlessly integrate basic research, drug discovery, and nonclinical and clinical development. Our turnkey and customized services include models for research and drug discovery; identifying targets and mechanisms of action; efficacy; pharmacology and pharmacokinetics; safety; and clinical research studies.
For additional information, please contact SRI Biosciences at biosciences@sri.com
[1] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3633606/
Research and development activities described were supported by:
- National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Number 1RC2AI087604-01 [P19559]
- Lawrence Berkeley National Lab (Berkeley Lab) Contract DE-AC02-05CH11231 [P21493]
- NIAID/NIH under Contract Number HHSN272201500013I [P23438]
- Biomedical Advanced Research and Development Authority (BARDA) under Contract Number IPIAA12OS99609
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Reference in this document to any specific commercial products, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government, the U.S. Department of Energy, or The Regents of the University of California or the Lawrence Berkeley National Laboratory. The views and opinions expressed in the case study do not necessarily state or reflect those of the United States Government, the U.S. Department of Energy, The Regents of the University of California or the Lawrence Berkeley National Laboratory.



