As the immune system’s equivalent of guided missiles, T-cells offer a seemingly ideal approach to disease management. They actively locate diseased cells and, after arriving at their target, exert a therapeutic effect proportionate to the disease burden. Unfortunately, many diseases such as cancer, autoimmune disorders and viral infections have immunological roots, and have become proficient at adapting to evade the immune system.
Current standard-of-care drugs for these diseases, such as small molecules or protein-based biologics, have their own drawbacks. These drugs are “passive” – unable to account for variations such as disease progression and patient size. As a result, many patients receive excessive drug doses that can lead to drug toxicity, or insufficient doses that can minimize efficacy and lead to drug resistance.
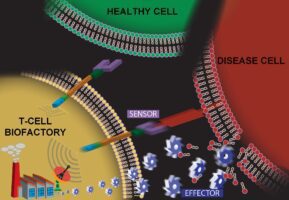
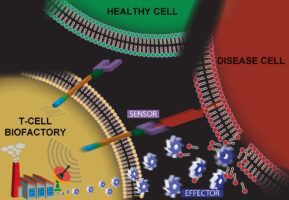
In recent years, SRI has received funding from the National Cancer Institute (NCI)* and the National Institute of Biomedical Imaging and Bioengineering (NIBIB)^ to implement research designed to maximize the disease-fighting potential inherent to the human immune system. Through immune T-cell engineering, our goal was to create and validate a platform of self-modulating T-cell-based “living-drug factories” that actively travel to disease sites, assess the disease burden, and modulate their own response to synthesize proportionate amounts of therapeutic peptides directly at the disease site.
Recently, Advanced Biosystems published a manuscript summarizing our team’s successful creation and initial validation of a T-cell-based platform technology that has potential to synthesize any protein after interacting with specific biomarkers on a diseased cell. At the heart of this technology is an artificial cell-signaling pathway that, when encoded into the genome of a T cell, transforms the cell into what we call a T-cell Biofactory. The T-cell Biofactory actively looks for diseased cells (because this is what T-cells inherently do!), triggering only when it actually engages with them. The Biofactory then co-opts the cellular machinery, autonomously regulating the synthesis of the protein that has been programmed as part of the artificial cell-signaling pathway. This localized release of the desired protein at the disease site will help prevent undesired side effects to healthy tissues.
SRI’s T-cell Biofactory, which represents an ideal example of synthetic biology, offers several advantages over existing and in-development approaches. The key differentiating factor is that components of the artificial cell-signaling pathway we’ve created are modular – meaning we can re-program the platform to target biomarkers on different diseased cells’ surface and then generate desired therapeutic proteins at the site of disease. This greatly expands its applicability for specific targeting and treatment of many cell-based diseases. Importantly, the T-cell Biofactory synthesizes and secretes therapeutic proteins proportionate to the number of antigen-presenting target cells, meaning that drug “doses” are autonomously calibrated to patients’ respective disease burden.
Having fully validated SRI’s proprietary approach with a reporter protein – which allowed us to visualize the T-cell Biofactory’s activity – our next step is to replace the reporter protein with a protein that has desired therapeutic properties. In addition, we will investigate methods to scale-up the production for T-cell Biofactory, and to assess its potency and specificity in in vivo studies.
We are thrilled with our team’s progress to date, particularly because it suggests the potential to replace passive drugs with those that actively search for a target disease, and then assemble complex biologics in the body. If successful, the T-cell Biofactory represents a new frontier in the treatment of immune system-based diseases.
SRI International is seeking enthusiastic Ph.D. scientists with experience in contemporary approaches to T-cell immunology, primary T-cell engineering, and/or immunological assays to support work on this promising CAR T-cell technology. Visit SRI’s careers page to learn about opportunities to contribute to the research team.
* National Cancer Institute Innovative Molecular Analysis Technologies program, which supports potentially transformative technologies.
^ National Institutes of Health (NIH) Director’s New Innovators Award, which is part of the NIH “High-Risk, High-Reward Research” program and supports unusually innovative research from early career investigators.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R21CA193064. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number DP2EB024245. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.” AND
“This work was funded by the National Institutes of Health through the NIH Director’s New Innovator Award Program, grant number 1DP2EB024245-01. Information on the New Innovator Program is at http://nihroadmap.nih.gov/newinnovator/