
Agile3DRx™
SRI’s scalable 3D inkjet printing and analysis platform for agile manufacturing of solid dosage pharmaceuticals
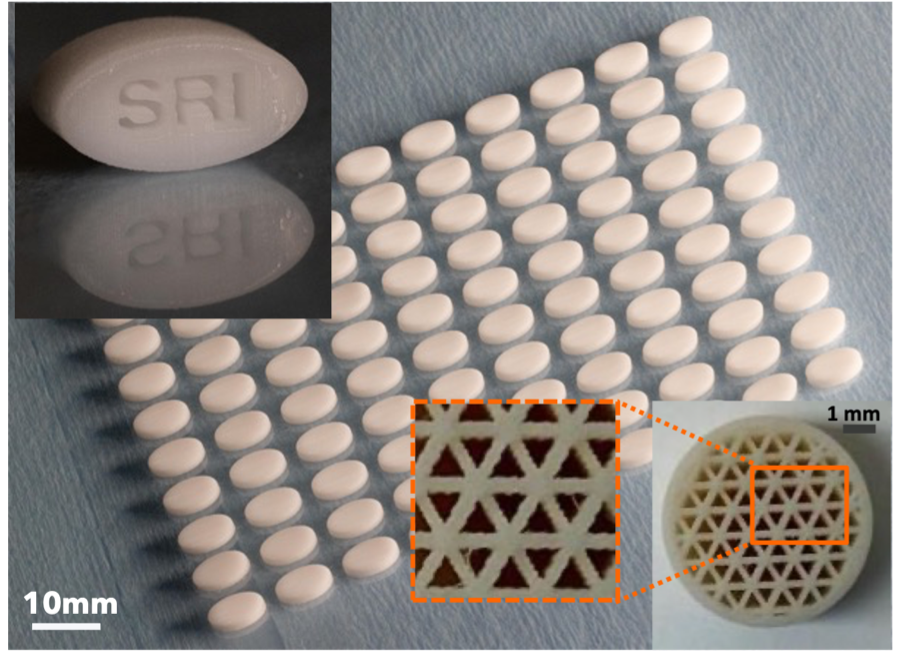
SRI’s Agile3DRx solid dosage printing system
SRI’s 3D inkjet printing technology, Agile3DRx, is a manufacturing technology platform for pharmaceutical solid dosage forms with significantly simplified processing steps and flexibility of throughput compared to current solid dose manufacturing. It supports production needs ranging from R&D and short runs to low- to medium-volume production.
This customizable and adaptable solid dose printing platform from SRI enables novel performance capabilities for the dosage form with easy digital implementation. Agile3DRx’s customization capability leads to on-demand and in-line implementation of strategies that can tailor tablet quality metrics for target drug product specifications. It also enables in-line metrology of the production process and quality control of the drug products for on-demand production.
Contact SRI to explore system customization, development collaboration, or licensing opportunities for solid dose pharmaceutical manufacturing.
Benefits of Agile3DRx
Summary:
- Fast turnaround time
- Adaptive production volumes, reduced scale up risk
- Rapid formulation iterations, compatible with small API quantities
- Cost-effective short runs for R&D or niche therapies
- On-demand/just-in-time manufacturing
- Innovation and high customization of dosage form and release profile
- In-line metrology compatible for reliable production and quality
Scalability
Agile3DRx overcomes the limitations of traditional pharmaceutical manufacturing, which is not well-suited for small or variable batch sizes often required in preclinical R&D, clinical trials, rare disease programs, or rapid formulation iterations.
The modular, scalable design enables production from single tablets to low- and medium-scale batches without needing major modifications to redesign/redevelop processes or systems. This flexibility allows the same manufacturing approach to be used across development phases, minimizing the need for bridging or crossover bioequivalence studies and significantly reducing development time and cost. Agile3DRx is compatible with small-volume active pharmaceutical ingredient (API) formulations and offers flexibility without complexity, allowing pharmaceutical developers to maintain process continuity across the drug development lifecycle, reduce validation overhead, and meet short-run production needs in a more agile and cost-effective way.
Speed and simplicity
The Agile3DRx system can expedite production timelines and simplify the lengthy, multi-step pharmaceutical production processes. It can also incorporate rapid formulation or dose changes and provide adaptive agile responses to emerging needs, for example, based on new pre-clinical or clinical data, significantly streamlining and speeding up drug development processes.
A fast change-over time (within hours) enabled by modular, disposable components can be realized. The system can be implemented as a deskside system or as a larger throughput system. Furthermore, no powder handling is required for SRI’s system, which significantly impacts ease of adoption and lowers the overall burden of facility requirements and establishment.
Customizable and digitally controlled
SRI’s solid dosage printing system offers easy customization of both the product and its manufacturing process, to help efficiently achieve the target quality metrics of the products.
Micron-size drop by drop resolution and digital customization enable several novel capabilities. Our digitally defined production process enables on-demand tuning of process parameters for various drug product characteristics (e.g., tailoring geometrical features, size, shape, dose, composition, and drug release characteristics).
On-demand production and supply chain resilience
Agile3DRx enables a more responsive, decentralized approach to the pharmaceutical supply chain. SRI’s system, with its small footprint, could meet the need for more agile manufacturing sites that are geographically dispersed, enabling just-in-time production to address clinical needs or sudden demand spikes without logistical delays. Just-in-time production reduces the cost and waste related to overproduction, transportation, and stockpiling.
Applications:
- Clinical trial material production
- Preclinical R&D and formulation iteration
- Early-stage development with limited API availability
- Orphan and rare disease drug programs
- Specialty medicines, fixed-dose combinations
- On-demand production
- Low stability formulations
Contact the Agile3DRx team
Learn more, request a demo, or explore whether Agile3DRx
fits your development goals and your application.